1. What is caprylohydroxamicacid?
Iron ions are famously restrictive when it comes to the growth of moulds, which is why caprylohydroxamicacid is a great choice as an organic acid for keeping them at bay. This substance efficiently chelates divalent and trivalent iron ions, meaning that mould growth is limited in such environments. Not only this, but the optimal length of its carbon chain also contributes to the degradation of cell membranes, resulting in its potent antimicrobial activity. Caprylohydroxamicacid is a unique alternative to preservatives and definitely worth considering.
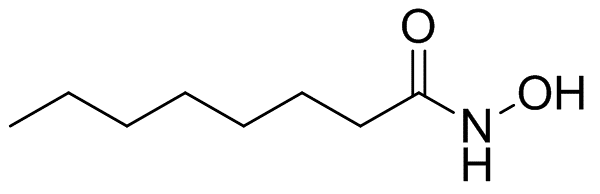
Caprylohydroxamicacid, an ester of the amino acid glycine, naturally occurs and can be synthetically produced too. It’s akin to arginine (another amino acid), but with a unique chemical structure. Discovered back in the 70s, this active component has proven its worth as a food preservative spanning four decades or so. And what’s more? It’s commonly acknowledged for being safe.

Caprylohydroxamic acid” – think about it as being part of around ten clusters. These sets can include both natural and fabricated esters, all known for their unique chemical structures, distinctive attributes, falling under the amphoteric classification (that “+” sign stands for amphoteric). Well now, this fascinating compound shows a remarkable knack for demolishing microorganisms. Yep. We’re talking troublemakers like Salmonella or Escherichia coli right through to Staphylococcus aureus and Candida albicans.
The primary reason it’s used as a germ-killing agent is because of its great ability to stop bacteria multiplying when pH levels are lower than 5.5, thanks in part to its special liking for glycine. And when pH values soar above 7.5? Well, due to its strong affinity for arginine, the story’s quite different – it displays potent opposition.
Let’s talk about caprylohydroxamic acid. The synthesis of this compound can arise from glycine or arginine, with the reaction conditions playing a key role in determining the source. In certain instances though, a completely different route is taken; protein synthesis through an artificial enzyme called octanoylglycine synthase (OGS). Interestingly enough, OGS expression happens within Azotobacter vinelandii and Pichia pastoris, specific categories of bacteria and archaea respectively. Now let’s switch gears for just a moment to chat about its use in vaccinations—pretty cool stuff! Caprylohydroxamic acid has achieved success when used against several bacterial infections such as Salmonella typhimurium , Streptococcus pneumoniae , Haemophilus influenzae and Neisseria meningitidis . Even more intriguing? It’s been employed instead of our go-to streptomycin in fights against Mycobacterium tuberculosis.
Let’s talk about caprylohydroxamic acid. The synthesis of this compound can arise from glycine or arginine, with the reaction conditions playing a key role in determining the source. In certain instances though, a completely different route is taken; protein synthesis through an artificial enzyme called octanoylglycine synthase (OGS). Interestingly enough, OGS expression happens within Azotobacter vinelandii and Pichia pastoris, specific categories of bacteria and archaea respectively. Now let’s switch gears for just a moment to chat about its use in vaccinations—pretty cool stuff! Caprylohydroxamic acid has achieved success when used against several bacterial infections such as Salmonella typhimurium , Streptococcus pneumoniae , Haemophilus influenzae and Neisseria meningitidis . Even more intriguing? It’s been employed instead of our go-to streptomycin in fights against Mycobacterium tuberculosis.
Let’s consider caprylohydroxamicacid’s inhibition potential. It shows a varied range when measured between pH 6-7; culminating in an astonishing 100% for Salmonella typhimurium and, surprisingly enough, zero effectiveness against Staphylococcus aureus. Now here is where the plot thickens – if we increase the concentrations right up to a ceiling of about 0.1%, this fascinating substance becomes more potent! Categorized as an top-notch alternative to antibiotics, caprylohydroxamicacid shines brightly due to its extraordinary efficacy—notably it even maintains this standard when given orally.
2. The benefits of using caprylohydroxamicacid
caprylohydroxamicacid (Octanoylox) is a highly effective, environmentally friendly and nontoxic antimicrobial agent. It has been used as an effective fungicide in food and pharmaceutical industries for the last 30 years. Some examples of its usage are:
- Can be used as an antifungal agent in agriculture, veterinary medicine, medicine
- Used to treat a number of infectious diseases such as certain bacterial infections, fungal infections and viral infections
- As a spoilage inhibitor in the food industry
3. How to use caprylohydroxamicacid
The absolute best pick for putting a stop to mould is nothing other than caprylohydroxamic acid. This particular blend, also known as CPA, is an intriguing concoction of desiccated coconut, capsicum and tea tree oil working in harmony. With its highly-praised antimicrobial property coupled with being entirely non-toxic makes it a top choice. And we can’t gloss over its commendably robust ability in curbing the growth of microbes now!
This particular chemical concoction finds usage in culinary goods as diverse as frosty ice creams, refreshing salad dressings, and creamy yogurts to silkened soy milk. Its main role? Ensuring the purity of such delectables by halting any trace of contamination right at its onset; it is known for its superb efficiency and effectiveness in stifling bacterial growth.
This particular chemical concoction finds usage in culinary goods as diverse as frosty ice creams, refreshing salad dressings, and creamy yogurts to silkened soy milk. Its main role? Ensuring the purity of such delectables by halting any trace of contamination right at its onset; it is known for its superb efficiency and effectiveness in stifling bacterial growth.

4. The side effects of using caprylohydroxamicacid
Revamped Text: “Ever heard of caprylohydroxamic acid? Well, it has quite the talent for killing microbes. In fact, its antimicrobial prowess makes it a snappy new substitute for preservatives; particularly handy when you’re looking to knock off those persistent fungi that adore residing on human skin. That’s probably why the American Academy of Dermatology advocates substituting your usual preservatives with this mighty compound! On the other hand, we’ve got our folks at British National Health Service who haven’t jumped onto this bandwagon just yet!

Utilizing Caprylhydroxamic acid ain’t without drawbacks, y’know. You got the gamut from as minor as parched skin to full-blown rashes and annoyance—and don’t even get me started on allergic reactions! Sure, you could sidestep these bad boys with a sunscreen packing SPF 30 or more—but is it worth the trouble? And for Pete’s sake— keep this caustic stuff clear of cuts, mucous membranes and especially those peepers; they ain’t gonna take kindly to irritation or any hindrance in healing. Oh, and did I forget something? Right, watch out—it might miff your respiratory system too.