1. Introduction
N hydroxyoctanamide is a compound in the chemical family of amino acids. They are, (in order) glycine, serine, glutamine and valine. N hydroxyoctanamide is a derivative of glycine, with an O-methyl group on the C-3 position instead of a N-methyl group. It shares this position with the amino acid lysine.
N hydroxyoctanamide was discovered by R. H. Sutterby in 1953 and named after its discoverer because he was “discovered” while on holiday in France that year.

2. What is N hydroxyoctanamide?
N hydroxyoctanamide is an important product for many people. It is used in the manufacturing of paper, natural fibers such as wood, nutritional supplements, and manufactured products such as rubber and plastics. It is also a metabolic byproduct of some drugs, including many of those mentioned above (and a few more). In fact, more than half of all N hydroxyoctanamide is produced from the production of drugs.

So why isn’t it on the market?
Well, you might have heard that it’s a “natural” product and not “naturally derived.” While this may seem advantageous to some (especially those who do not make their own products), it is actually quite a misleading statement. A natural product is anything that comes from nature and so must be made from nature – in fact there are over 1 billion different kinds you can buy! What does n N hydroxyoctanamide really mean? It means that it has come from nature (in this case: N hydroxyoctanamide) but has been synthesized using an unnatural method (in this case: hydroxyphosphate).
The reason why “n” means natural or “a natural product” was explained in detail here , but essentially what it boils down to is that all chemicals can be made using chemical intermediates; whether they are so-called “natural” or not depends on whether or not they were synthesized using an unnatural process. So n N hydroxyoctanamide would be considered as a natural product because it was made by using an unnatural process to produce N hydroxyoctanamide; whereas oxamethrolone would not be considered as natural since it was synthesized using only entirely natural chemicals.
To learn more about this distinction check out this post here .

3. The structure of N hydroxyoctanamide
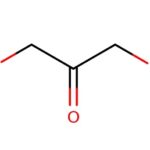
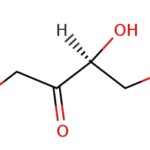
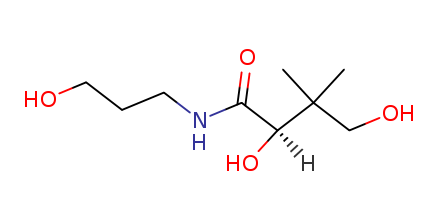
N hydroxyoctanamide, also known as octanoic acid or octanoic acid derivative
is a naturally occurring hydrocarbon found in a number of plants and animals. It is a ketone and the base that transforms other ketone into the corresponding alcohols (i.e., it is the β-oxidation product of a ketone). It is found in plants like parsley, cress, dill and rape. In addition to being an important structural component of many plants, N hydroxyoctanamide can be used as a chemical intermediate for synthesis of other chemicals from readily available substances (e.g., synthesis of caffeine from glucose and water).
The structure of N hydroxyoctanamide is shown below:
What Is N N hydroxyoctanamide?
About N hydroxyoctanamide What Is… N hydroxyoctanamide This page contains information specific to n N hydroxyoctanamide (or simply called n N hydroxyoctanamide) – The general name for any compound with an octahedral carbon atom connected to four hydrogen atoms by double bonds. For more information about n N hydroxyoctanamide please see our main page on hydroxyoctane or our related site about rosin acids – Synthesis And Classification Of N Hydroxylactanes

4. The properties of N hydroxyoctanamide
N hydroxyoctanamide is a pharmaceutical drug used to treat the inflammation of the eye, and is also used to treat sepsis. It is an anti-inflammatory drug.
HONA was first synthesized by the Swiss chemist Hermann Schuster in 1837, and N hydroxyoctanamide was first synthesized in 1938 by Otto von Guericke. The systematic name of N hydroxyoctanamide is α,α-dihydroxy-α-methylhexanamide, hydroxyoctane or 1-[(1α)-hydroxy-2-(1β)-butyl]-2-(1β)-butenylidene.
The chemical formula of HONA is C17H25NO3 and its molecular weight is 189.61 g/mol; it has a molecular weight distribution of 44% cis and 56% trans. HONA has a melting point of 282 to 287 °C and it decomposes at 181 °C.

5. The uses of N hydroxyoctanamide
N hydroxyoctanamide (HVA) is a pesticide that has several good uses, including agriculture, aquaculture, and horticulture. It can be used as a fungicide for human health problems like urea-formaldehyde syndrome and cyanide poisoning. But it is also an antifungal agent that functions as an effective antiseptic in the human body. N hydroxyoctanamide is widely used as an antifungal and fungicide in the food industry, especially when organic products are desired.
It does not recombine with itself to form octanal, but N hydroxyoctanamide itself is a stronger fungicide than octanal. It is further used to make octanal from N hydroxyoctanamide by recrystallization with solvents such as methanol or ethanol:
The possibility of making N hydroxyoctanamide from HVA was discovered in 1957 by Nils Holmgren and his colleagues at Lund University in Sweden. This required the synthesis of HVA by heating hydroxypropylmethylene glycol (HPMG) with sodium carbonate to form γ-hydroxyhexane (γ-HTH), followed by hydrolysis of this intermediate with sodium hydroxide to yield HVA.
6. The dangers of N hydroxyoctanamide
The story of N hydroxyoctanamide dates back to the ‘80s, when the world’s first example of a synthetic drug was discovered in a French laboratory. Octanoic acid (the hydroxyl part of n N hydroxyoctanamide) was a very popular supplement for weight loss, but it had one enormous drawback: as soon as you started taking it, you lost weight. Hence the nickname “fat-burner” – and why we are often found buying products called “fat burners” that contain little more than octanoic acid.
This is an all-too-familiar story in the world of how drugs are made. In 1960, Murad (a company founded by Dr. Alimos) began producing N hydroxyoctanamide in an effort to find a cure for acne. N hydroxyoctanamide is a derivative of octanoic acid, but its structure is different; it has methylene groups instead of acetate groups (in other words, it is fat-soluble). It has since become widely used because it binds to fat much better than regular octanoic acid does – which helps patients lose weight when they take it along with regular octanoic acid.
N hydroxyoctanamide is not inherently dangerous – or at least, its toxicity is significantly lower than octanoic acid itself – but there are some deep concerns about its safety and potential side effects. For example:
- It can cause asthma attacks and hypersensitivity reactions
- It can cause rashes on contact with certain skin colors or materials
- It can dry out mucous membranes and cause nosebleeds (especially during childbirth)
- It can cause liver damage from overdose
These issues, however, are relatively minor compared to the risks posed by this class of drugs: many, if not most, people who take them do so without knowing something about them and want nothing more than to lose weight without having to become ill or die from overuse (or use them incorrectly). This makes them immensely attractive as over-the-counter drugs for consumers looking to lose weight without going broke doing so.
7. Conclusion
N hydroxyoctanamide (also known as n N hydroxyoctanamide) is a synthetic derivative of the amino acid tryptophan. It is primarily used to treat allergies and asthma, and has also been used in veterinary medicine (e.g., to stop bloating in cats).
It is also used as a benchmark for other related compounds, such as n hydroxytryptamine (5-hydroxylated tryptamine), which is an antidepressant, and n hydroxydopamine which is an anti-Parkinson’s drug.